Hydrofunctionalizations of Alkenes
Nitrogen-containing compounds, such as amines, enamines, and imines are valuable and commercially important bulk chemicals, specialty chemicals, and pharmaceuticals. Classical methods for the synthesis of amines, either on laboratory or industrial scale, generally require multiple steps involving refined starting materials. The hydroamination and hydroaminoalkylation reactions represent two new promising alternative approaches for the atom-economical synthesis of these valuable products in an environmentally benign, waste-free fashion directly from simple alkenes.
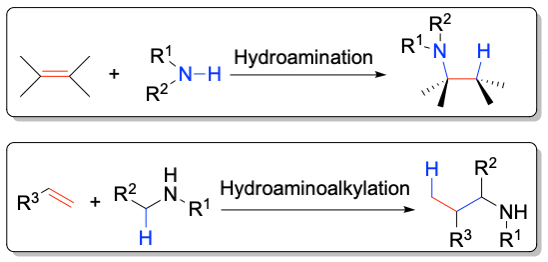
The Hultzsch group has developed a series of chiral catalyst systems based on early transition metals (e.g. Zr, Ta) and rare earth metals (e.g. Sc, Y, La, Lu) for the hydroamination reaction. For example, 3,3‘-substituted binaphtholate rare earth metal complexes catalyze the intramolecular hydroamination of aminoalkenes with enantiomeric excess exceeding 90% ee.
Chem. Commun. 2004, 730-731
J. Am. Chem. Soc. 2006, 128, 3748-3759
Chem. Eur. J. 2009, 15, 12819-12827
Organometallics 2018, 37, 4358–4379
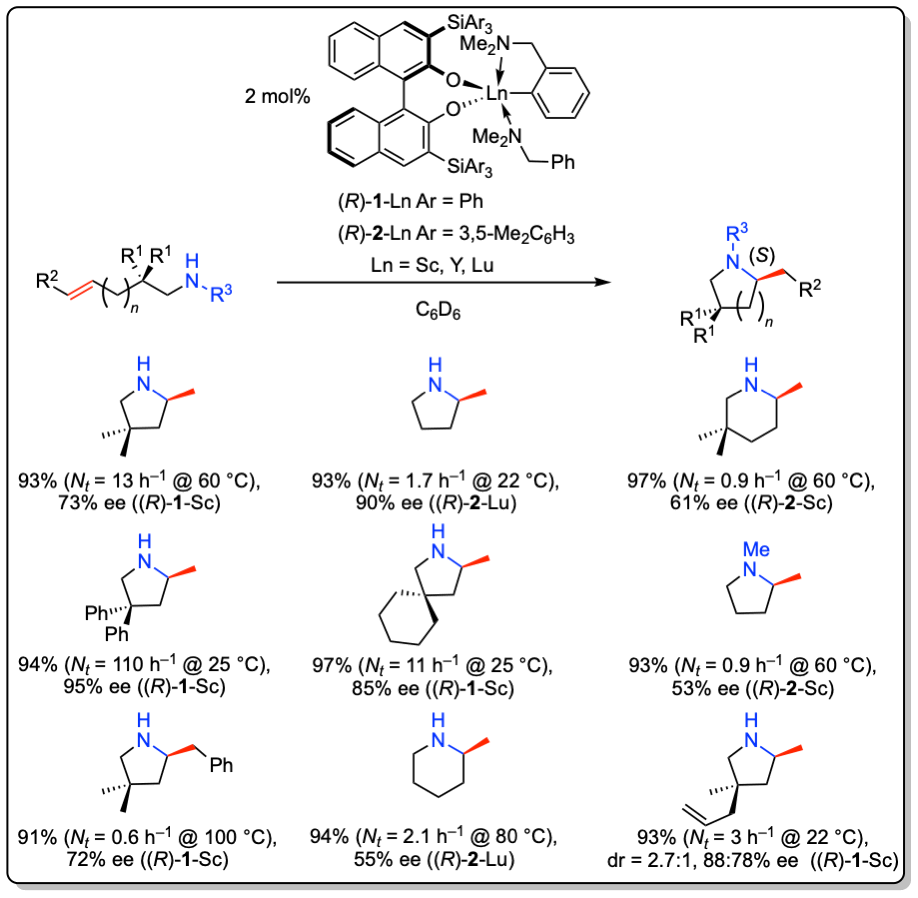
In addition, these complexes are the first catalysts for the asymmetric intermolecular hydroamination of non-activated alkenes with simple amines with enantioselectivities of up to 61% ee.
Angew. Chem. Int. Ed. 2010, 49, 8984-8987
Organometallics 2013, 32, 1394-1408
Organometallics 2018, 37, 4358–4379
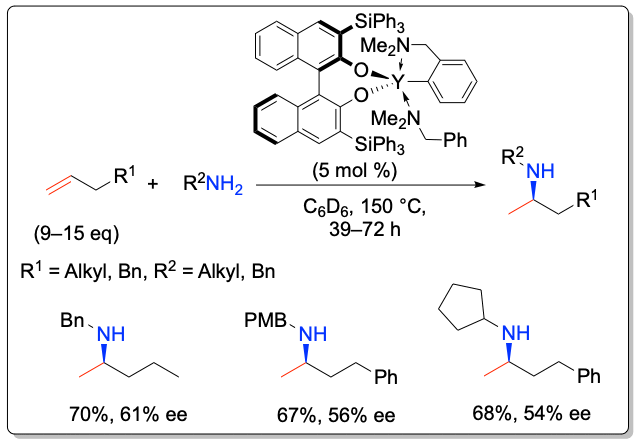
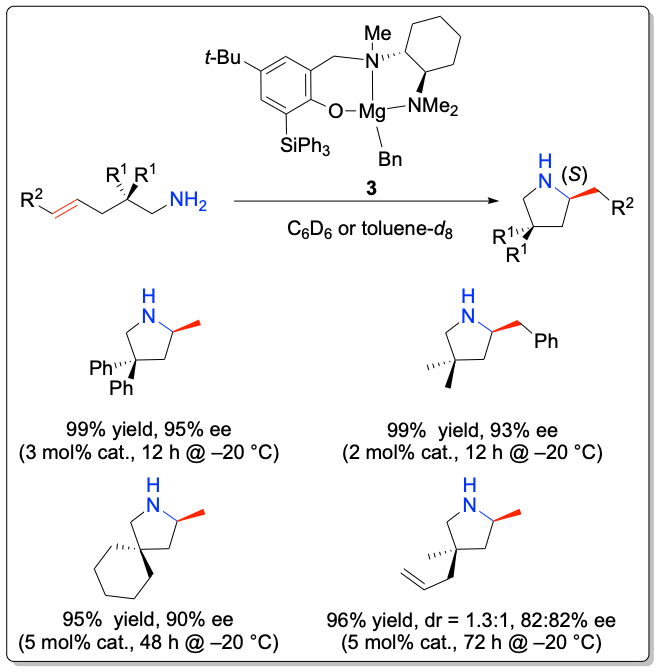
While most studies have focused on the development of transition metal-based catalyst systems, main group metal complexes of the alkaline and alkaline earth metals lend themselves for catalyst development thanks to their low toxicity. Significant challenges represent the labile metal-ligand interactions of main group metals, which often lead to facile Schlenk-type ligand redistribution processes, thwarting efforts to perform transformations in a stereoselective manner. In addition to the first chiral lithium-based catalysts for the asymmetric hydroamination, the Hultzsch group has developed the first highly enantioselective chiral phenoxyamine magnesium catalyst system that combines outstanding reactivity at temperatures as low as –20 °C and high enantioselectivities of up to 93% ee in hydroamination/cyclization reactions of aminoalkenes. The high catalytic activity of this system also allowed to perform the first magnesium-catalyzed intermolecular hydroamination of vinyl arenes with amines with high anti-Markovnikov selectivity.
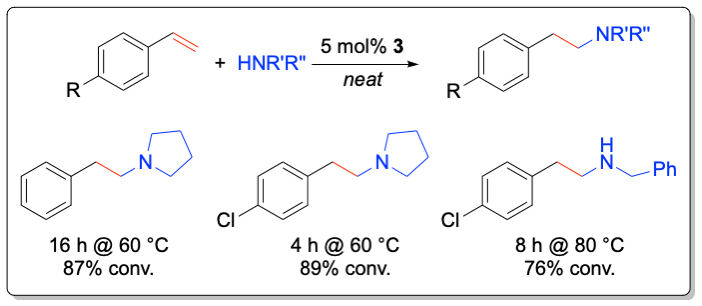
Angew. Chem. Int. Ed. 2012, 51, 394-398
A series of 3,3’-substituted binaphtholate niobium and tantalum complexe catalyze the hydroaminoalkylation with high chemoselectivity and enantiomselectivities of up to 98% ee. The hydroaminoalkylation proceeds via C-C bond formation between an amine and an alkene, in which the α-C-H bond of the amine is being activated. This C-H activation is in contrast to the amine N-H bond activation observed in the hydroamination reaction.
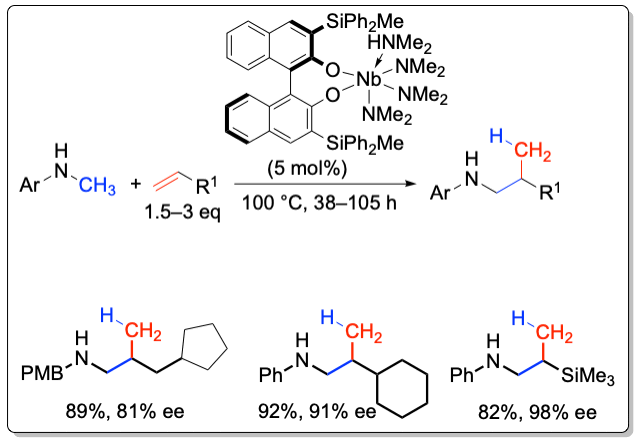
Organometallics 2011, 30, 921-924
J. Am. Chem. Soc. 2012, 134, 3300-3311
