Borrowing Hydrogen Strategy
Another sustainable strategy for the atom-efficient synthesis of N-containing molecules examined in our group, is the borrowing hydrogen (BH) methodology This strategy became a topic of great interest as it can be applied for various transformations of cheap and harmless starting materials, most commonly used are alcohols. In general, this reaction sequence leads to the formation of new carbon- carbon or carbon- nitrogen bonds and involves three main steps. In the first step the substrate gets activated through dehydrogenation (oxidation), then a functionalization reaction (e.g. N-alkylation, Aldol reaction, Michael additions) takes place. To complete this cascade reaction, the formed product gets hydrogenated (reduced) returning the hydrogen which was generated in the first step. The overall process is redox neutral and after the completed reaction sequence there is no gain or loss of hydrogen, avoiding the application of further reducing agents or external hydrogen.
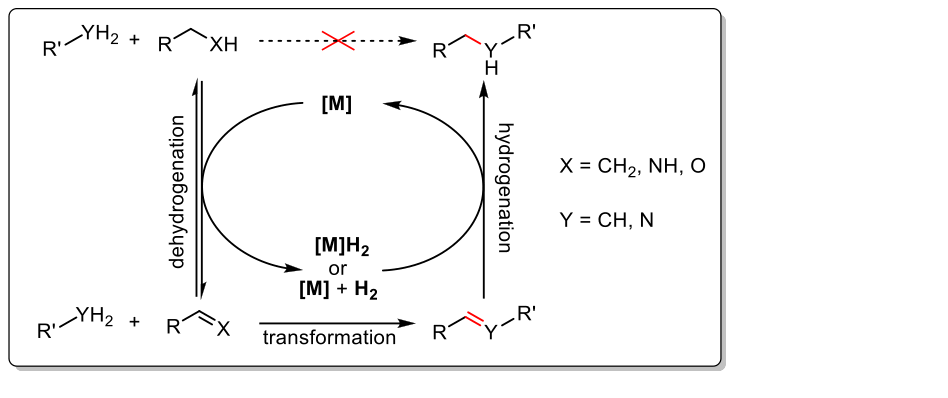
In recent years, the development of abundant metal complexes based on manganese, iron and cobalt became more and more important. The use of these metals in catalysis is particularly attractive due to their abundance in the earth crust. Above all, pincer complexes draw attention showing outstanding potential in hydrogen transfer reactions. Therefore, one part of our research focuses on the synthesis of novel pincer-based catalytic systems. In this regard, a highly active Mn(I) catalyst based on a nonsymmetric PN3-ligand scaffold for the N-alkylation of amines with alcohols utilizing the borrowing hydrogen methodology was developed. The combination of low catalyst loading and mild reaction conditions provides high efficiency for this atom-economic transformation based on metal-ligand cooperation.
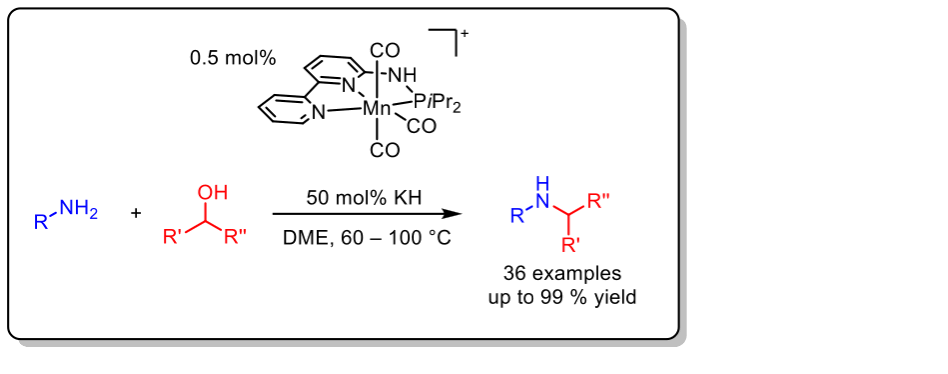
Org. Lett. 2019, 21, 3142–3147.
The second part deals with the development of new reaction cascades. Hence, a one-pot three-component condensation of alcohols, amines and phosphites to form a-N-alkylamino phosphonates was developed using the nitrile-ligated iron-based Knölker’s complex in combination with a chiral BINOL-based phosphoric acid. The base-free N-alkylation of anilines with benzylic alcohols can be switched in favor of imine formation simply by chaning from a closed to an open reaction system. Further functionalization of the in situ synthesized imine leads to a-N-alkylamino phosphonates in an atom-economic fashion.
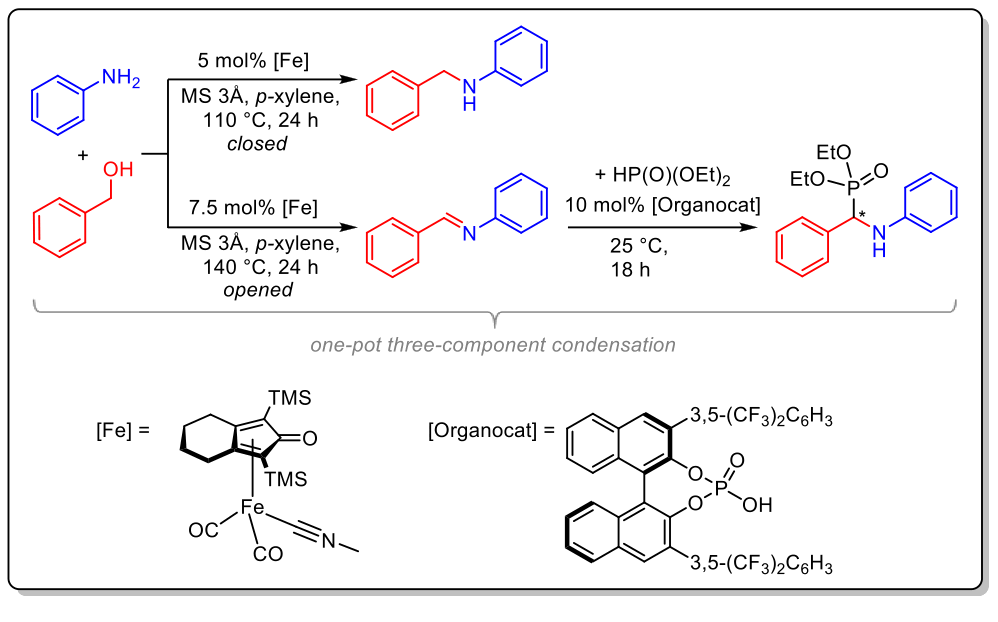
Eur. J. Org. Chem. 2019, 3105–3111.
